Saturday, November 30, 2013
Gases Lab Test
Hello, I hope you all had a nice break. I was wondering if you guys had any ideas for the gases lab test--I feel like we're supposed to use a eudiometer like we did for the last lab, but I can't figure out how to get the mystery gas from the tank to the eudiometer. Does anyone know how to solve this or have any better ideas?
Monday, November 25, 2013
Partner Quiz and Review
Hey everyone!
Today in class, we went over the worksheet we had for homework over the weekend. The answers to 'Gas Stoichiometry 1' worksheet are..
1. 2.3 L of H2
2. C3H8 + 5O2 ---> 3CO2 + 4H2O and 22.7 L of CO2
3. 2KClO3 ---> 3O2 + 2KCl and 0.12 g KClO3
As Mrs. Friedmann stated in class, there will be a study session tomorrow morning at 7am for all those who would like to attend. Don't forget to bring a charged calculator for the test tomorrow!! The video going over the study packet is now in the Unit 5 box.
We also took a partner quiz that we are now able to use for studying purposes. Hope everyone did a good job! :) The key to this quiz is in the Unit 5 Keys folder- so don't forget to take a look at that too.
Good luck studying everyone!
Today in class, we went over the worksheet we had for homework over the weekend. The answers to 'Gas Stoichiometry 1' worksheet are..
1. 2.3 L of H2
2. C3H8 + 5O2 ---> 3CO2 + 4H2O and 22.7 L of CO2
3. 2KClO3 ---> 3O2 + 2KCl and 0.12 g KClO3
As Mrs. Friedmann stated in class, there will be a study session tomorrow morning at 7am for all those who would like to attend. Don't forget to bring a charged calculator for the test tomorrow!! The video going over the study packet is now in the Unit 5 box.
We also took a partner quiz that we are now able to use for studying purposes. Hope everyone did a good job! :) The key to this quiz is in the Unit 5 Keys folder- so don't forget to take a look at that too.
Good luck studying everyone!
Saturday, November 23, 2013
Molar Volume Lab and Gas Stoich
Hello My Fellow Classmates!
Homework for the Weekend:
- Finish the Molar Volume Lab for Monday
- Complete Gas Stoich Worksheets (under Unit 5 Handouts)
- Continue studying for the Unit 5 Test on Tuesday by working on the Review Packet!
In class, we discussed a lot about the stoichiometry that is involved with finding molar volumes and gas pressures. We also talked more about STP (Standard Temperature and Pressure). You can find the notes on these topics in the Unit 5 section under Unit 5 notes!
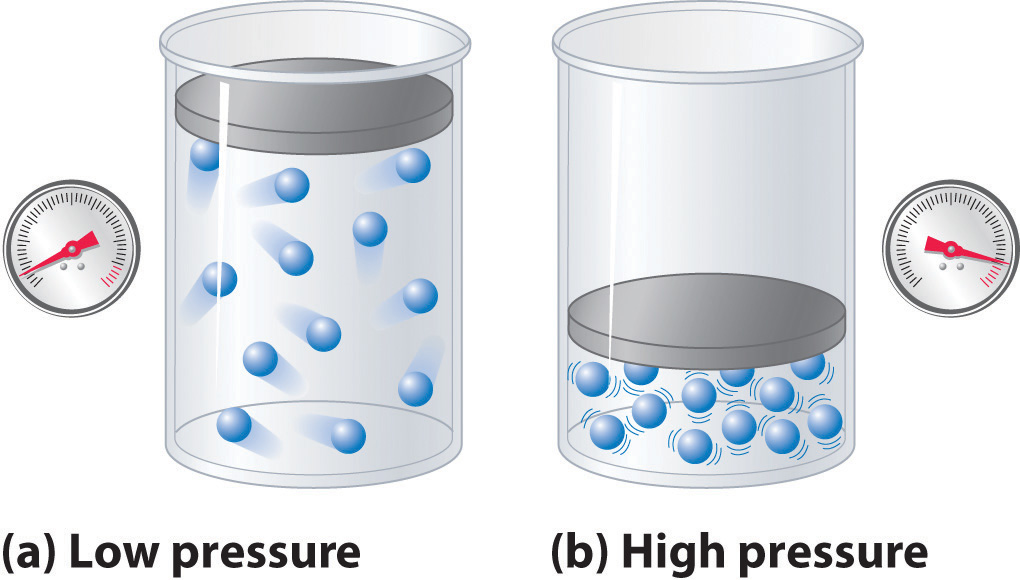
Question of the Day:
Jordan asked a very great question in class on Friday and we had a demonstration that displayed some of it.
"Does a gas ever exert no/0 pressure?"
Mrs. Friedmann explained that all gases will exert some pressure, but never 0. This is because particles in gases are constantly moving and bouncing off of one another as well as surroundings. This is what defines a gas. In order to have 0 pressure, the particles would have to stop moving altogether. If the particles stopped moving, they wouldn't form a gas anymore. A gas at "Absolute 0" wouldn't be a gas, but a solid.
Overall, Study tons and tons for the test!
Next Scribe: Elayna M. :)
Homework for the Weekend:
- Finish the Molar Volume Lab for Monday
- Complete Gas Stoich Worksheets (under Unit 5 Handouts)
- Continue studying for the Unit 5 Test on Tuesday by working on the Review Packet!
In class, we discussed a lot about the stoichiometry that is involved with finding molar volumes and gas pressures. We also talked more about STP (Standard Temperature and Pressure). You can find the notes on these topics in the Unit 5 section under Unit 5 notes!
Question of the Day:
Jordan asked a very great question in class on Friday and we had a demonstration that displayed some of it.
"Does a gas ever exert no/0 pressure?"
Mrs. Friedmann explained that all gases will exert some pressure, but never 0. This is because particles in gases are constantly moving and bouncing off of one another as well as surroundings. This is what defines a gas. In order to have 0 pressure, the particles would have to stop moving altogether. If the particles stopped moving, they wouldn't form a gas anymore. A gas at "Absolute 0" wouldn't be a gas, but a solid.
Overall, Study tons and tons for the test!
Next Scribe: Elayna M. :)
Thursday, November 21, 2013
Lab: Molar Volume
Post Author: Georgia A.
Homework:
Homework:
1) 2 WebAssigns are due tonight @ 11:59pm
2) Watch the video going over the prelab problems for the lab you did today, and take notes (check in tomorrow). The write-up for this lab is due Monday, 11/25.
3) Print out a copy of the Unit 5 Review Packet (in the Unit 5 Handouts folder) and start work on it tonight! Our unit test is on Tuesday, 11/26.
4) If you were uncertain about the manometer calculations you did for homework last night, check your work against the key posted in the Unit 5 Keys folder
Lab
Background and Purpose:
The "molar volume" of a gas is the amount of space one mole of a gas occupies at Standard Temperature (0℃) and Standard Pressure (1.0 atm). Standard Temperature and Pressure are abbreviated "STP." As it turns out, this number is always the same: one mole of gas (ANY gas) at STP always occupies 22.4 litters. In this lab you will attempt to demonstrate this fact.
Pre-Lab Questions:
Reaction of 0.0285 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen gas. The gas was collected by water displacement in a 22.0℃ water bath. The barometric pressure in the lab was 746.4 mmHg.
1. Use Dalton's law and the vapor pressure of water at 22.0℃ to calculate the partial pressure of hydrogen gas in the tube.
2. Use the Combined Gas Law to calculate the corrected volume of hydrogen at STP
3. What is the theoretical number of moles of hydrogen that can be produced from 0.0285 g of Mg?
4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to calculate the molar volume of hydrogen at STP.
Lab Write-up
1. Title
2. Purpose
3. Data
4. Calculations: Use data from both trials
a. Write and balance the reaction between magnesium and hydrochloric acid
b. Calculate the mass of magnesium and then calculate the moles of magnesium for each of the two trials (Recall that 1.00 cm of magnesium ribbon has a mass of 0.01085 grams)
c. Using stoichiometry, calculate the moles of H2 produced for each trial.
d. Using Dalton's Law of Partial Pressures, calculate the pressure of the H2 gas in the eudiometer for each trial.
e. Using the Combined Gas Law, calculate the volume of the H2 gas at STP for each trial.
f. Calculate the molar volume of a gas at STP for each trial. Average the two molar volumes to get the final molar volume you will report in your conclusion
g. Calculate the percent error of your reported molar volume.
h. In setting up this experiment, a student noticed that a bubble of air leaked into the eudiometer. What effect would this have on the measured volume of a hydrogen gas? Would the calculated molar volume of hydrogen be too high or low?
Conclusion
a. Claim - state here what you found the molar volume of the hydrogen gas to be.
b. Evidence - What data led you to this conclusion? Talk through the logical steps that led you from the data you measured in class to the claim stated above.
c. Reasoning - How close were you to the actual molar volume of a gas? What was your percent error? What SPECIFIC mistakes in the lab could have caused the error in your results?
Data:
Observations:
Observations:
It starts bubbling from the Magnesium and Copper at the bottom of the eudiometer. Bubbles rush faster as time progresses. The gas is pushing the H2O out of the rubber stopper. A space full of Hydrogen gas starts forming at the top.
Next Author: Pamela H.
Tuesday, November 19, 2013
More About Gases
Homework:
We started off class going over homework from the night before (the 2 gas problems worksheets), and then Mrs. Friedmann handed back the Gas Variables packet that the class did on Friday which we're supposed to go over by ourselves tonight. You should correct it using the key posted in Unit 5 Keys folder on Moodle. The other homework tonight is a small worksheet about pressure, and we don't have to do the 2 worksheets with Manometer problems! :)
In Class:
We started off with a lecture on Flexible vs. Inflexible Containers:
Flexible Containers:
example: a balloon
-pressure stays constant
-volume changes
Inflexible Container:
example: metal tank
-volume stays the same
-pressure changes
Then Mrs. Friedmann introduced Direct and Indirect/Inverse Relationships to us:
A direct relationship is when one property/value increases the other increases too. Or if it decreases, the other one decreases as well.
An indirect relationship is when one property/value increases and the other decreases or vice versa.
So in applying this to the Ideal Gas Law (PV=nRT), when you solve for the constant R by writing R=PV/nT it's useful to know that PV (and other variables next to each other) have an inverse/indirect relationship while variables that are on top of each other like V/T have a direct relationship.
And that's it as far as lecturing goes! For the rest of the day we just did some really cool demos!
Liquid Nitrogen Demo:
We'd all been wondering what was up with the small styrofoam container in the corner pouring what looked like steam for a good part of the class, so when Mrs. Friedmann pulled it out we were all pretty anxious to see what it was exactly. What we definitely weren't expecting was for Mrs. Friedmann to pull out 15 inflated balloons from the 6 inch wide, 6 inch long container. Turns out, it was filled with a substance called liquid nitrogen which can freeze pretty much about anything almost instantly, including Peter Dales backpack. Thankfully, he remembered to take his chromebook out beforehand, but he probably had an interesting time trying to bite into his sandwich during lunch.
THIS IS NOT A GOOD IDEA

Liquid Oxygen:
Mrs. Friedmann gave us a quick demo on liquid oxygen, too. Apparently, liquid oxygen is very powerful oxidizing agent and can ignite quickly from very small sources of heat. This was demonstrated when Mrs. Friedmann collected a small amount of it into a test tube and inserted a small stick with a single spark at the tip into the tube. The spark ignited, and there was a brief flare.
We started off class going over homework from the night before (the 2 gas problems worksheets), and then Mrs. Friedmann handed back the Gas Variables packet that the class did on Friday which we're supposed to go over by ourselves tonight. You should correct it using the key posted in Unit 5 Keys folder on Moodle. The other homework tonight is a small worksheet about pressure, and we don't have to do the 2 worksheets with Manometer problems! :)
In Class:
We started off with a lecture on Flexible vs. Inflexible Containers:
Flexible Containers:
example: a balloon
-pressure stays constant
-volume changes
Inflexible Container:
example: metal tank
-volume stays the same
-pressure changes
Then Mrs. Friedmann introduced Direct and Indirect/Inverse Relationships to us:
A direct relationship is when one property/value increases the other increases too. Or if it decreases, the other one decreases as well.
An indirect relationship is when one property/value increases and the other decreases or vice versa.
So in applying this to the Ideal Gas Law (PV=nRT), when you solve for the constant R by writing R=PV/nT it's useful to know that PV (and other variables next to each other) have an inverse/indirect relationship while variables that are on top of each other like V/T have a direct relationship.
And that's it as far as lecturing goes! For the rest of the day we just did some really cool demos!
Liquid Nitrogen Demo:
We'd all been wondering what was up with the small styrofoam container in the corner pouring what looked like steam for a good part of the class, so when Mrs. Friedmann pulled it out we were all pretty anxious to see what it was exactly. What we definitely weren't expecting was for Mrs. Friedmann to pull out 15 inflated balloons from the 6 inch wide, 6 inch long container. Turns out, it was filled with a substance called liquid nitrogen which can freeze pretty much about anything almost instantly, including Peter Dales backpack. Thankfully, he remembered to take his chromebook out beforehand, but he probably had an interesting time trying to bite into his sandwich during lunch.
THIS IS NOT A GOOD IDEA

Liquid Oxygen:
Mrs. Friedmann gave us a quick demo on liquid oxygen, too. Apparently, liquid oxygen is very powerful oxidizing agent and can ignite quickly from very small sources of heat. This was demonstrated when Mrs. Friedmann collected a small amount of it into a test tube and inserted a small stick with a single spark at the tip into the tube. The spark ignited, and there was a brief flare.
Monday, November 18, 2013
The Combined Gas Law
Answers to Yesterday's Homework
First thing in class today Mrs. Friedmann checked in our homework and went over the answers. The answers are as follows:
1) .75 L
2) 27 degrees Celsius
3) .12 moles
4) a) .021 moles
b) 120 g/mole (divide the mass of freon by the number of moles of freon)
5) .0560 moles
6) 240 K or -33 degrees Celsius (either would be acceptable)
7) 58.1 moles
8) 1.88 moles
9) 3.42 minutes (this problem was just for fun and will not be on any exams)
Work: 1.72 * 10^-4 moles O2 * 1 h * 1000007 cockroaches = 2.01 moles O2
1 cockroach * h 60 minutes
First thing in class today Mrs. Friedmann checked in our homework and went over the answers. The answers are as follows:
1) .75 L
2) 27 degrees Celsius
3) .12 moles
4) a) .021 moles
b) 120 g/mole (divide the mass of freon by the number of moles of freon)
5) .0560 moles
6) 240 K or -33 degrees Celsius (either would be acceptable)
7) 58.1 moles
8) 1.88 moles
9) 3.42 minutes (this problem was just for fun and will not be on any exams)
Work: 1.72 * 10^-4 moles O2 * 1 h * 1000007 cockroaches = 2.01 moles O2
1 cockroach * h 60 minutes
Combined Gas Law Notes
Next, we took notes on the combined gas law. Mrs. Fridmann's notes can be found here: http://gbs-moodle.glenbrook225.org/moodle/file.php/12015/1314_Unit_5/Unit_5_Notes/11.18_Class_Notes.pdfChemistry Demos
Finally, at the end of class we watched Ms. Friedmann show us some neat chemistry demos. It's too bad if you missed them, but (like videos of farting cats and Hollywood celebrities) they can both be found on the internet.
Homework
Tonight's homework is the "Gas Laws" handouts. We did the first one in class. It looks like this:
Good luck and have a good night!
The next blogger will be Lauren B.
Labels:
11/18/13,
Combined Gas Law,
Gas Laws,
Q2 Brandon Moy
Sunday, November 17, 2013
The Gas Unit
Gasses, and more Gasses
Recap of Friday November 15,
- Received a packet on properties of gasses
- Completed the packet with partners
- Had two tape-in worksheets assigned
Explanation:
The substitute handed out a review packet on the behavior of gasses which you can find in the handouts section under Unit 5 on moodle. The packet was due at the end of class, and if you finished you were to begin the homework which was two pages in your journal.
Homework:
- Two worksheets in your notebook
Next Scribe: Brandon Moy
Friday, November 15, 2013
Return of the Tests!
The test grades are out!
Here is what we did today (11/14/13)
- Got back the tests
- Went over any questions needed
- Discovered some fantastic failures
Mrs. Friedmann handed out the tests today, and stating that there was an unexpected amount of great failures. Each student had a chance to ask about a question, row by row. We clarified Net Ionic Equations and the limiting reaction equation on the free response. Mrs. Friedmann also said that any students who put A for question 14 would get the point since it was unclear what the actual answer was. That raised some of the students grades up.
Homework
Since the day was full of review of the test there wasn't much to write about. Mrs. Friedmann assigned a ChemThink along with notes that are due tomorrow. You were also supposed to watch the Youtube Video of Mr. Arum Majumdar as he will be coming to speak with us next Thurdsay. You are to write a question on a notecard to him, addressed with your name, Mrs. Friedmann's name, and with your period (5).
If you cant find the video on moodle here it is:
Best wishes to Mrs. Friedmann and her mom!
Wednesday, November 13, 2013
Colligative Properties
Video:
We started off the class with a video on soda slushies. Later in the class we learned why this works.
Colligative property notes:
These kind of properties are physical properties of solutions that depend on how many solute particles are present, NOT on what the solute is.
Aqueous solution equations:
molecular- C6H12O6(s) ---> C6H12O6(aq) 1 mole particles
ionic- NaCl(s)---->Na+(aq) + Cl-(aq) 2 moles particles
This is why ionic compounds are more effective for colligative properties.
Two colligative properties:
The two main properties will focus on are Boiling Point Elevation and Freezing Point Depression.
Boiling Point:
The ions of a solute block the water from escaping. The water requires more energy to escape the container and so the boiling point is raised.
Freezing Point:
When a solid is formed, it can be thought of a crystal forming. More solute in a solution makes the formation of these crystals require more energy. Thus, the freezing point is lowered.
Solubility of Solids v. Gases:
As we as learned, a solid's solubility is increased when the temperature is increased. With gases however, the solubility decreases with an increase in temperature. Another way to affect the solubility of gases is with pressure. By increasing the pressure of a gas, the solubility increases.
Also, I'm sorry that this is late.
The next scribe is:
Daniel W.
We started off the class with a video on soda slushies. Later in the class we learned why this works.
Colligative property notes:
These kind of properties are physical properties of solutions that depend on how many solute particles are present, NOT on what the solute is.
Aqueous solution equations:
molecular- C6H12O6(s) ---> C6H12O6(aq) 1 mole particles
ionic- NaCl(s)---->Na+(aq) + Cl-(aq) 2 moles particles
This is why ionic compounds are more effective for colligative properties.
Two colligative properties:
The two main properties will focus on are Boiling Point Elevation and Freezing Point Depression.
Boiling Point:
The ions of a solute block the water from escaping. The water requires more energy to escape the container and so the boiling point is raised.
Freezing Point:
When a solid is formed, it can be thought of a crystal forming. More solute in a solution makes the formation of these crystals require more energy. Thus, the freezing point is lowered.
Solubility of Solids v. Gases:
As we as learned, a solid's solubility is increased when the temperature is increased. With gases however, the solubility decreases with an increase in temperature. Another way to affect the solubility of gases is with pressure. By increasing the pressure of a gas, the solubility increases.
Also, I'm sorry that this is late.
The next scribe is:
Daniel W.
Saturday, November 9, 2013
Molarity & Stoichiometry Review
Scribe Post Author: Serene P.
11/8/13
To begin class today, Ms. Friedmann checked our previous night's homework assignments. Then, she informed us of our homework over the weekend.
1) Unit 4 Test has been moved to Wednesday!
-A study session will be held Wednesday morning at 7 am
- A review packet was given and a set of extra precipitation stoich problems (however, these are optional preparation items for the test)
2) Read sections 10.3 and 10.4 in your textbook and take notes in your journal-due Tuesday
-You need to know about Colligative Properties for the test, but you do not need to know how to calculate freezing point depression, boiling point elevation, or osmotic pressure.
3) WebAssign 10.3-due Monday night at 11:59 pm
During class, we also picked up a sheet titled, "Chemistry Scene Investigation~Trouble in the Chemistry Store Room." However, we were not able to get to this activity.
We went over the two homework sheets. Ms. Friedmann demonstrated how to do question 2a on the homework sheet titled 'Molarity.' Here is the work for the question:
2) What is the molarity of each ion present in aqueous solutions prepared by dissolving 15.00g of the following compounds in water to make 5.25 L of solution?
a) magnesium bromide?
15.00g MgBr2 x 1 mole MgBr2 = 0.08148 moles MgBr2
184.10g MgBr2
0.08148 moles MgBr2 = 0.0155 M MgBr2 ←overall chemical molarity
5.25 L Solution
Chemical concentration x number of particles
So…
0.0155 x 1M Mg2+ ions = 0.0155M
0.0155 x 2M Br- ions = 0.031 M
If you want the total ion concentration, do 0.0155 x 3 = 0.0465M
For question one of the ‘Molarity’ worksheet, the answer was 6.46M NH3. The work is shown below:
11.0g NH3 x 1 mole NH3 = 0.64554 moles NH3
17.04g NH3
100 mL x 1 L = 0.1 L
1000mL
0.64554 moles NH3 = 6.46 M NH3
0.1 L NH3
On the ‘Concentration and Solution Stoichiometry’ sheet, the answer for question one should have been 0.519 M NaOH. To solve this question, you should have done the total number moles in solution divided by the total volume of the solution. The answer was a little closer to the higher molarity.
For question two on this worksheet, the answer was that both reactants were used up equally. There was not any limiting reactant because the amount that resulted from both Pb(NO3)2 and KI was 0.0875g PbI2.
The remaining answers for questions 2b, 2c, and 3 on the ‘Molarity’ worksheet will be found on the key that should be posted on moodle.
Ms. Friedmann also discussed the differences of molecular compounds and ionic compounds getting dissolved in water. She explained that the ionic compounds will be able to split into more particles when dissolved in water. This is because the water molecules will be able to attach to the ends of particles with opposing charges. An example is MgBr2.
Ionic compounds are able to conduct electricity as well.
However, molecular compounds in water will act differently. They will not conduct electricity and will not break apart. One particle of the compound will become one particle in solution. However, from being a solid, the compound will be referred to as aqueous because water gets in between molecules of the compound. It will separate molecules of the compound, not ions. Ionic compounds will separate into ions when in solution. 
We also learned how colligative properties depend on the number of particles in solution. Water boils at a higher temperature when particles are added. Particles with charges that hold onto water molecules keep them from escaping into a gaseous state. Therefore, you must increase the heat energy to reach the boiling point of the water(boiling point elevation). If there is a lot of solute present, the water can boil at a temperature higher than a 100 degrees.
At the end of class, we had some fun with Georgia’s large hot pack. 
The next scribe post author will be Colin S.
Thursday, November 7, 2013
More Solution Stoichiometry
Fun with Homework
Fun with Molarity
Next, Mrs. Friedmann passed back our Concentration and Molarity Simulation packets. We went over these, and the key is also posted on the moodle page under Unit 4 Keys.
Fun with Supersaturation
After that, Mrs. Friedmann showed us the demonstration of the supersaturated sodium acetate solution. The sodium acetate packet was put in boiling water to heat it up so the the water could dissolve all of the sodium acetate. Then the packet was slowly cooled down until it became supersaturated. The liquid was very syrupy and slow moving. When Mrs. Friedmann flipped the metal disk around that was inside the packet, the sodium acetate went out of solution and crystalized. The packet became warm because all of the heat energy we put into it when putting it in the boiling water is now being released. Below is a video of this happening:
More Fun with Homework
This brought us up to the end of class. Our homework for tonight is the two worksheets that were handed out in class today which are the Molarity Thinking Problems worksheets. These can be found in the Unit 4 handouts folder. If you are struggling with these problems, post them on the blog! Try to check the blog again by 10 PM and see if you can answer anyone's questions.
Fun with Blogging
The next blogger is...Valerie K
Wednesday, November 6, 2013
Solutions & Stoichiometry
It's finally here... Solutions + stoichometry!
Okay, so you might not be so excited about this. But hopefully this blog
can clear up any confusions you have about blending what we already
know about stoichometry with this unit's topic: solutions.
- Today, we started class by turning in our Molarity Stiumlations packets, which we're on schedule to go over tomorrow. If you need one of these, they're in the Unit 4 Handouts folder!
- Then, we started on the torture part of this unit: Solution stoich.
Here's a brief overview of the notes we took and the problems we did!
Disclaimer: Ms. Friedmann's notes, which she wrote in class today, are in the Unit 4 Notes folder
under 'Notes on Solution Stoichiometry'. Go check these out, as well.
First, we redefined stoichometry: looking at one "thing" in a reaction (either a reactant or a product) and figuring out how much of another "thing" in the same reaction will be used up or produced based on the measurement of "thing" 1.
This definition might seem a little extensive, but to remind everyone...
Now, we all remember doing (a little bit too many of) these problems!
So, where do solutions come in?
Well, to solve stoichometry problems involving Molarity, we need new algorithms.
Obviously, the new parts of this algorithm are:
- Calculations using the volume to find other measurements. Use the molarity to convert from volume to moles, and vice versa.
- Calculations using the molarity to find other measurements. Use the volume to convert from molarity to moles, and vice versa.
Let's do a problem:
This is the first problem on the worksheet entitled 'solution stoich', located in the Unit 4 Handouts folder.
CaCl2 (aq) + 2 AgNO3 (aq) -----> Ca(NO3)2 (aq) + 2 AgCl (s)
What volume of 0.150 M AgNO3 is needed to react with 45.0 mL of 1.50 M CaCl2?
What is "thing 1"? CaCl2, because we are given its molarity and volume.
What is "thing 2"? AgNO3, because we are asked to calculate the volume of it needed to react.
There are two ways to start this problem, either with the molarity or the volume of CaCl2. We chose to start with the volume of CaCl2.
Since the volume is in mL, we must convert to L just by shifting the decimal 3 places to the left.
Knowing our first conversion factor must be 1.50 moles CaCl2/1L, because molarity is moles of solute over liters of solvent, we can begin to set this problem up.
0.045 L * 1.50 moles CaCl2/1L
Now, we need to convert from moles of CaCl2 to moles of AgNO3. To do that, we look at the balanced equation. There are 2 moles of AgNO3 for every 1 mole of CaCl2. So...
0.045 L * 1.50 moles CaCl2/1L * 2 moles AgNO3/1 mole CaCl2
Next, we need to get from moles to volume of AgNO3. So, knowing the molarity of AgNO3 and using the corresponding conversion factor...
0.045 L * 1.50 moles CaCl2/1L * 2 moles AgNO3/1 mole CaCl2 * 1L/0.150 moles AgNO3 =
0.90 L of AgNO3!
And that's it! The rest of this sheet, along with the two others recieved in class today, are for homework. Good luck, and feel free to ask questions in the comments section!
Here's an awesome Khan Academy video on solution stoichiometry if you're still confused:
Next scribe is Mary L!
Tuesday, November 5, 2013
So, What is Molarity Anyway?
So, What is Molarity Anyway?
Scribe: Grace K.
Date: November 5th, 2013
We all know it's a bummer when you can't make it to Mrs. Friedmann's fifth period class. It is the best class of the day after all! However, don't worry about the content you missed. This blog will get you all caught up on everything you need to know!
Handouts
As usual, Mrs. Friedmann had an assortment of handouts for us to pick up when we walked into class today. These included a packet titled Concentration and Molarity PhET-Chemistry Labs and a notes sheet on how to mix a solution given a value of molarity properly. Both can either be found using the links included here or in the Unit 4 Handouts and Unit 4 Notes, respectively.
Last Night's Homework
Mrs. Friedmann checked in the homework due for today in our journal. This included two molarity worksheets that can either be found by this link or in the Moodle handouts folder and the molarity packet that we began in class yesterday.
At this time, we spent a few minutes reviewing question 21 in the Molarity Packet. An in depth explanation can be found in Mrs. Friedmann's key. The main idea is this: molarity compares moles of solute to liters of SOLUTION. This solution consists of both the solute and the solvent-NOT JUST THE SOLVENT! So, in question 21, the mistake was that the student needed 50mL of solution, not 50 mL of water. We will explore this concept further in the simulation section.
The BIG Idea
The in-class notes today were on the main ideas of molarity. These notes can be found either by this link or in the Unit 4 Moodle notes folder. Here's a video to help you understand this very important topic.
Mixing Things Up
After we understood, this whole "molarity" thing, we decided to "mix things up a bit". It was at this point we looked at the handout on how to mix solutions given the value of molarity. This can be found either by this link or in the Unit 4 Moodle notes folder.
The handout prompts, "Suppose you work in a chemistry lab. Your boss tells you to make 0.50 liters of a 0.75 M solution of sodium chloride. How would you do it??". Mrs. Friedmann gave us five minutes with a partner to discuss possible solutions and this is what the class came up with:
Class Brainstormed Solution:
Step 1: Find moles of sodium chloride.
Step 2: Convert moles to grams so we can actually measure it in the lab.
Step 3: Add water until you get 0.50 Liters.
As it turns out, we are a very smart class and our solution was very correct! A more in depth answer can be found on the answer key. Some students in the class noted that they used the proportionality approach to compute the calculations. However, Mrs. Friedmann stated that she encourages us to use dimensional analysis, as it will be more useful to us in the long run.
"I won't believe it until I see it!"
To make sure we understood the process explored in the notes above, Mrs. Friedmann performed a simulation for the class. She first put 22 grams of sodium chloride in a small container, by zeroing the container on the scale and measuring the substance accordingly. She filled a volumetric flask (see below) with less than 5oo mL of water and added the 22 grams of sodium chloride. Mrs. Friedmann shook the mixture to allow it to dissolve. She also added water to assist in the dissolving process, being sure she did not add too much water to the mixture as such a mistake is hard to reverse. It is best practice to eye the volumetric flask from the level of the meniscus, to make sure one is as accurate as possible. Right before our eyes, Mrs. Friedmann had created 0.50 liters of a 0.75 M solution of sodium chloride, using our calculation of grams of solute necessary.
Let Us Not Whine About Wine...
Volumetric flasks happen to be one of Mrs. Friedmann's favorite pieces of equipment. (I smell a potential Christmas present! A bejeweled volumetric flask perhaps? ) Today, we learned that the device is apparently used by some people in their kitchen to decant wine. We also learned a bit on the process of wine oxidation. Volumetric flasks are meant to be created very exactly. Once a potential flask is made, it is filled with highly concentrated water. A special machine laser marks the meniscus of the water at an exact measurement, ensuring accuracy. You learn something new everyday!
Last Night's Molarity Homework
Towards the end of the period, we spent a bit of time discussing the Molarity calculations we performed in our journal. The answer key can be found by this link or in the Unit 4 Moodle keys folder. Here are some ideas that may help you to understand this worksheet.
1c. Remember that molecular compounds don't dissociate. The only way to show solid sugar vs. sugar water is using the aqueous symbol (aq) and the solid symbol (s).
3c. Remember that if given mL of solution, you must convert to L using a conversion factor.
6 and 7. These problems are known as dilution problems. This equation will be very useful in solving problems such as these:
M1 x V1 = M2 x V2
....where M stands for molarity and V stands for volume.
SIG FIGS: When computing calculations of this liking (given a quantity of solute and solution), use the significant figures from the measurement with the smallest number of significant figures given.
Homework
1) Complete the Concentration and Molarity PhET Simulation Packet (in the Unit 4 Handouts folder). You will need to click on the link posted in the Unit 4 box to access the simulations...the link will take you to a page with 8 or 9 simulations; you will only need to access the ones called "Concentration" and "Molarity". Due tomorrow.
2) WebAssign 10.2 - Solubility. Due tonight by 11:59 pm.
THE NEXT SCRIBE IS CAMERON B.
THE NEXT SCRIBE IS CAMERON B.
Subscribe to:
Comments (Atom)