Saturday, November 30, 2013
Gases Lab Test
Hello, I hope you all had a nice break. I was wondering if you guys had any ideas for the gases lab test--I feel like we're supposed to use a eudiometer like we did for the last lab, but I can't figure out how to get the mystery gas from the tank to the eudiometer. Does anyone know how to solve this or have any better ideas?
Monday, November 25, 2013
Partner Quiz and Review
Hey everyone!
Today in class, we went over the worksheet we had for homework over the weekend. The answers to 'Gas Stoichiometry 1' worksheet are..
1. 2.3 L of H2
2. C3H8 + 5O2 ---> 3CO2 + 4H2O and 22.7 L of CO2
3. 2KClO3 ---> 3O2 + 2KCl and 0.12 g KClO3
As Mrs. Friedmann stated in class, there will be a study session tomorrow morning at 7am for all those who would like to attend. Don't forget to bring a charged calculator for the test tomorrow!! The video going over the study packet is now in the Unit 5 box.
We also took a partner quiz that we are now able to use for studying purposes. Hope everyone did a good job! :) The key to this quiz is in the Unit 5 Keys folder- so don't forget to take a look at that too.
Good luck studying everyone!
Today in class, we went over the worksheet we had for homework over the weekend. The answers to 'Gas Stoichiometry 1' worksheet are..
1. 2.3 L of H2
2. C3H8 + 5O2 ---> 3CO2 + 4H2O and 22.7 L of CO2
3. 2KClO3 ---> 3O2 + 2KCl and 0.12 g KClO3
As Mrs. Friedmann stated in class, there will be a study session tomorrow morning at 7am for all those who would like to attend. Don't forget to bring a charged calculator for the test tomorrow!! The video going over the study packet is now in the Unit 5 box.
We also took a partner quiz that we are now able to use for studying purposes. Hope everyone did a good job! :) The key to this quiz is in the Unit 5 Keys folder- so don't forget to take a look at that too.
Good luck studying everyone!
Saturday, November 23, 2013
Molar Volume Lab and Gas Stoich
Hello My Fellow Classmates!
Homework for the Weekend:
- Finish the Molar Volume Lab for Monday
- Complete Gas Stoich Worksheets (under Unit 5 Handouts)
- Continue studying for the Unit 5 Test on Tuesday by working on the Review Packet!
In class, we discussed a lot about the stoichiometry that is involved with finding molar volumes and gas pressures. We also talked more about STP (Standard Temperature and Pressure). You can find the notes on these topics in the Unit 5 section under Unit 5 notes!
Question of the Day:
Jordan asked a very great question in class on Friday and we had a demonstration that displayed some of it.
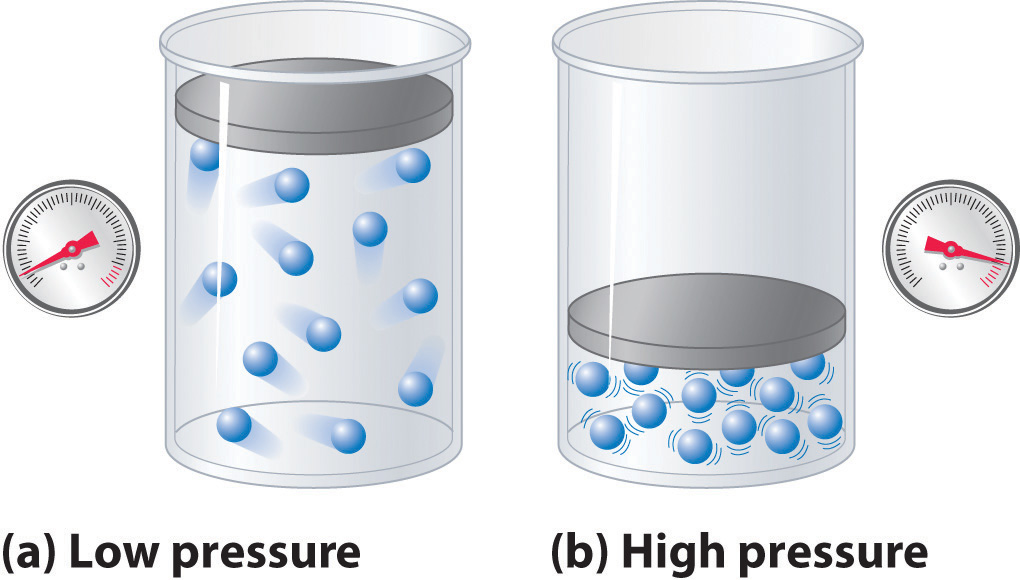
"Does a gas ever exert no/0 pressure?"
Mrs. Friedmann explained that all gases will exert some pressure, but never 0. This is because particles in gases are constantly moving and bouncing off of one another as well as surroundings. This is what defines a gas. In order to have 0 pressure, the particles would have to stop moving altogether. If the particles stopped moving, they wouldn't form a gas anymore. A gas at "Absolute 0" wouldn't be a gas, but a solid.
Overall, Study tons and tons for the test!
Next Scribe: Elayna M. :)
Homework for the Weekend:
- Finish the Molar Volume Lab for Monday
- Complete Gas Stoich Worksheets (under Unit 5 Handouts)
- Continue studying for the Unit 5 Test on Tuesday by working on the Review Packet!
In class, we discussed a lot about the stoichiometry that is involved with finding molar volumes and gas pressures. We also talked more about STP (Standard Temperature and Pressure). You can find the notes on these topics in the Unit 5 section under Unit 5 notes!
Question of the Day:
Jordan asked a very great question in class on Friday and we had a demonstration that displayed some of it.
"Does a gas ever exert no/0 pressure?"
Mrs. Friedmann explained that all gases will exert some pressure, but never 0. This is because particles in gases are constantly moving and bouncing off of one another as well as surroundings. This is what defines a gas. In order to have 0 pressure, the particles would have to stop moving altogether. If the particles stopped moving, they wouldn't form a gas anymore. A gas at "Absolute 0" wouldn't be a gas, but a solid.
Overall, Study tons and tons for the test!
Next Scribe: Elayna M. :)
Thursday, November 21, 2013
Lab: Molar Volume
Post Author: Georgia A.
Homework:
Homework:
1) 2 WebAssigns are due tonight @ 11:59pm
2) Watch the video going over the prelab problems for the lab you did today, and take notes (check in tomorrow). The write-up for this lab is due Monday, 11/25.
3) Print out a copy of the Unit 5 Review Packet (in the Unit 5 Handouts folder) and start work on it tonight! Our unit test is on Tuesday, 11/26.
4) If you were uncertain about the manometer calculations you did for homework last night, check your work against the key posted in the Unit 5 Keys folder
Lab
Background and Purpose:
The "molar volume" of a gas is the amount of space one mole of a gas occupies at Standard Temperature (0℃) and Standard Pressure (1.0 atm). Standard Temperature and Pressure are abbreviated "STP." As it turns out, this number is always the same: one mole of gas (ANY gas) at STP always occupies 22.4 litters. In this lab you will attempt to demonstrate this fact.
Pre-Lab Questions:
Reaction of 0.0285 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen gas. The gas was collected by water displacement in a 22.0℃ water bath. The barometric pressure in the lab was 746.4 mmHg.
1. Use Dalton's law and the vapor pressure of water at 22.0℃ to calculate the partial pressure of hydrogen gas in the tube.
2. Use the Combined Gas Law to calculate the corrected volume of hydrogen at STP
3. What is the theoretical number of moles of hydrogen that can be produced from 0.0285 g of Mg?
4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to calculate the molar volume of hydrogen at STP.
Lab Write-up
1. Title
2. Purpose
3. Data
4. Calculations: Use data from both trials
a. Write and balance the reaction between magnesium and hydrochloric acid
b. Calculate the mass of magnesium and then calculate the moles of magnesium for each of the two trials (Recall that 1.00 cm of magnesium ribbon has a mass of 0.01085 grams)
c. Using stoichiometry, calculate the moles of H2 produced for each trial.
d. Using Dalton's Law of Partial Pressures, calculate the pressure of the H2 gas in the eudiometer for each trial.
e. Using the Combined Gas Law, calculate the volume of the H2 gas at STP for each trial.
f. Calculate the molar volume of a gas at STP for each trial. Average the two molar volumes to get the final molar volume you will report in your conclusion
g. Calculate the percent error of your reported molar volume.
h. In setting up this experiment, a student noticed that a bubble of air leaked into the eudiometer. What effect would this have on the measured volume of a hydrogen gas? Would the calculated molar volume of hydrogen be too high or low?
Conclusion
a. Claim - state here what you found the molar volume of the hydrogen gas to be.
b. Evidence - What data led you to this conclusion? Talk through the logical steps that led you from the data you measured in class to the claim stated above.
c. Reasoning - How close were you to the actual molar volume of a gas? What was your percent error? What SPECIFIC mistakes in the lab could have caused the error in your results?
Data:
Observations:
Observations:
It starts bubbling from the Magnesium and Copper at the bottom of the eudiometer. Bubbles rush faster as time progresses. The gas is pushing the H2O out of the rubber stopper. A space full of Hydrogen gas starts forming at the top.
Next Author: Pamela H.
Tuesday, November 19, 2013
More About Gases
Homework:
We started off class going over homework from the night before (the 2 gas problems worksheets), and then Mrs. Friedmann handed back the Gas Variables packet that the class did on Friday which we're supposed to go over by ourselves tonight. You should correct it using the key posted in Unit 5 Keys folder on Moodle. The other homework tonight is a small worksheet about pressure, and we don't have to do the 2 worksheets with Manometer problems! :)
In Class:
We started off with a lecture on Flexible vs. Inflexible Containers:
Flexible Containers:
example: a balloon
-pressure stays constant
-volume changes
Inflexible Container:
example: metal tank
-volume stays the same
-pressure changes
Then Mrs. Friedmann introduced Direct and Indirect/Inverse Relationships to us:
A direct relationship is when one property/value increases the other increases too. Or if it decreases, the other one decreases as well.
An indirect relationship is when one property/value increases and the other decreases or vice versa.
So in applying this to the Ideal Gas Law (PV=nRT), when you solve for the constant R by writing R=PV/nT it's useful to know that PV (and other variables next to each other) have an inverse/indirect relationship while variables that are on top of each other like V/T have a direct relationship.
And that's it as far as lecturing goes! For the rest of the day we just did some really cool demos!
Liquid Nitrogen Demo:
We'd all been wondering what was up with the small styrofoam container in the corner pouring what looked like steam for a good part of the class, so when Mrs. Friedmann pulled it out we were all pretty anxious to see what it was exactly. What we definitely weren't expecting was for Mrs. Friedmann to pull out 15 inflated balloons from the 6 inch wide, 6 inch long container. Turns out, it was filled with a substance called liquid nitrogen which can freeze pretty much about anything almost instantly, including Peter Dales backpack. Thankfully, he remembered to take his chromebook out beforehand, but he probably had an interesting time trying to bite into his sandwich during lunch.
THIS IS NOT A GOOD IDEA

Liquid Oxygen:
Mrs. Friedmann gave us a quick demo on liquid oxygen, too. Apparently, liquid oxygen is very powerful oxidizing agent and can ignite quickly from very small sources of heat. This was demonstrated when Mrs. Friedmann collected a small amount of it into a test tube and inserted a small stick with a single spark at the tip into the tube. The spark ignited, and there was a brief flare.
We started off class going over homework from the night before (the 2 gas problems worksheets), and then Mrs. Friedmann handed back the Gas Variables packet that the class did on Friday which we're supposed to go over by ourselves tonight. You should correct it using the key posted in Unit 5 Keys folder on Moodle. The other homework tonight is a small worksheet about pressure, and we don't have to do the 2 worksheets with Manometer problems! :)
In Class:
We started off with a lecture on Flexible vs. Inflexible Containers:
Flexible Containers:
example: a balloon
-pressure stays constant
-volume changes
Inflexible Container:
example: metal tank
-volume stays the same
-pressure changes
Then Mrs. Friedmann introduced Direct and Indirect/Inverse Relationships to us:
A direct relationship is when one property/value increases the other increases too. Or if it decreases, the other one decreases as well.
An indirect relationship is when one property/value increases and the other decreases or vice versa.
So in applying this to the Ideal Gas Law (PV=nRT), when you solve for the constant R by writing R=PV/nT it's useful to know that PV (and other variables next to each other) have an inverse/indirect relationship while variables that are on top of each other like V/T have a direct relationship.
And that's it as far as lecturing goes! For the rest of the day we just did some really cool demos!
Liquid Nitrogen Demo:
We'd all been wondering what was up with the small styrofoam container in the corner pouring what looked like steam for a good part of the class, so when Mrs. Friedmann pulled it out we were all pretty anxious to see what it was exactly. What we definitely weren't expecting was for Mrs. Friedmann to pull out 15 inflated balloons from the 6 inch wide, 6 inch long container. Turns out, it was filled with a substance called liquid nitrogen which can freeze pretty much about anything almost instantly, including Peter Dales backpack. Thankfully, he remembered to take his chromebook out beforehand, but he probably had an interesting time trying to bite into his sandwich during lunch.
THIS IS NOT A GOOD IDEA

Liquid Oxygen:
Mrs. Friedmann gave us a quick demo on liquid oxygen, too. Apparently, liquid oxygen is very powerful oxidizing agent and can ignite quickly from very small sources of heat. This was demonstrated when Mrs. Friedmann collected a small amount of it into a test tube and inserted a small stick with a single spark at the tip into the tube. The spark ignited, and there was a brief flare.
Monday, November 18, 2013
The Combined Gas Law
Answers to Yesterday's Homework
First thing in class today Mrs. Friedmann checked in our homework and went over the answers. The answers are as follows:
1) .75 L
2) 27 degrees Celsius
3) .12 moles
4) a) .021 moles
b) 120 g/mole (divide the mass of freon by the number of moles of freon)
5) .0560 moles
6) 240 K or -33 degrees Celsius (either would be acceptable)
7) 58.1 moles
8) 1.88 moles
9) 3.42 minutes (this problem was just for fun and will not be on any exams)
Work: 1.72 * 10^-4 moles O2 * 1 h * 1000007 cockroaches = 2.01 moles O2
1 cockroach * h 60 minutes
First thing in class today Mrs. Friedmann checked in our homework and went over the answers. The answers are as follows:
1) .75 L
2) 27 degrees Celsius
3) .12 moles
4) a) .021 moles
b) 120 g/mole (divide the mass of freon by the number of moles of freon)
5) .0560 moles
6) 240 K or -33 degrees Celsius (either would be acceptable)
7) 58.1 moles
8) 1.88 moles
9) 3.42 minutes (this problem was just for fun and will not be on any exams)
Work: 1.72 * 10^-4 moles O2 * 1 h * 1000007 cockroaches = 2.01 moles O2
1 cockroach * h 60 minutes
Combined Gas Law Notes
Next, we took notes on the combined gas law. Mrs. Fridmann's notes can be found here: http://gbs-moodle.glenbrook225.org/moodle/file.php/12015/1314_Unit_5/Unit_5_Notes/11.18_Class_Notes.pdfChemistry Demos
Finally, at the end of class we watched Ms. Friedmann show us some neat chemistry demos. It's too bad if you missed them, but (like videos of farting cats and Hollywood celebrities) they can both be found on the internet.
Homework
Tonight's homework is the "Gas Laws" handouts. We did the first one in class. It looks like this:
Good luck and have a good night!
The next blogger will be Lauren B.
Labels:
11/18/13,
Combined Gas Law,
Gas Laws,
Q2 Brandon Moy
Sunday, November 17, 2013
The Gas Unit
Gasses, and more Gasses
Recap of Friday November 15,
- Received a packet on properties of gasses
- Completed the packet with partners
- Had two tape-in worksheets assigned
Explanation:
The substitute handed out a review packet on the behavior of gasses which you can find in the handouts section under Unit 5 on moodle. The packet was due at the end of class, and if you finished you were to begin the homework which was two pages in your journal.
Homework:
- Two worksheets in your notebook
Next Scribe: Brandon Moy
Subscribe to:
Posts (Atom)