We had a sub today for our quiz. We were given the whole period for the quiz, but if you finished early you were to start working on the Coulombic Attraction Packet, which was due Monday
12/16/13
To start class:
Mrs. Friedman reminded us that because we are being given less help this chapter, there will not be a video posted before the test. The test is on Thursday and the review will be Wednesday in the morning instead of Thursday.
After that, we went over our homework. Note: for #7 of page 4, you couldn't find the mass number because you weren't given the number of neutrons. A key thing to remember about the Coulombic Attraction Packet is that as the distance between the electron(s) and the proton(s) increases, the force of attraction decreases. In order to increase the force of attraction while the distance stays the same, the number of protons must increase.
Trends in the Periodic Table
1) Atomic Radius (aka atomic size)
2) Ionization energy
3) Electronegativity
Today we only got through the trend of atomic size. The notes are posted on moodle, but here is the basis of it:
a) As you move down the columns of the periodic table, the size of atoms increase.
Ex. A hydrogen atom is smaller than a lithium atom, which is smaller than a sodium atom.



b) As you move from left to right on the periodic table, the size of atoms decrease. This is because the added protons will increase the force of attraction and make the atom more compact. The added electrons remain on the same energy level as the previous farthest electrons and will space out from the other electrons, thereby adding little force to compete with the nucleus.
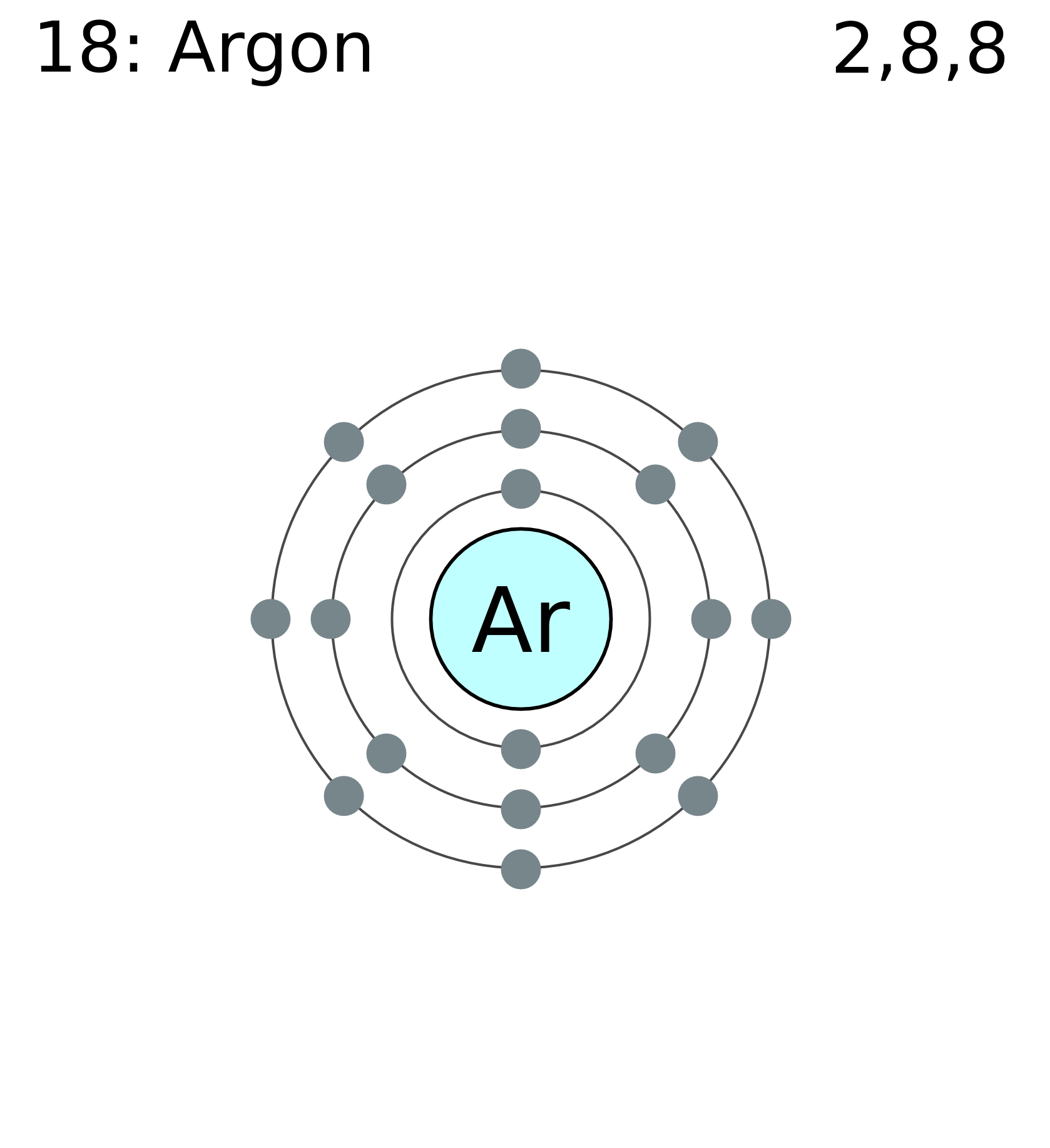
Ex. A sodium atom is smaller than a silicon atom, which is smaller than a argon atom.

Homework:
The review packet is due tomorrow.
Finish the 4 sheets that were handed out today (on moodle)
Check your Coulombic Attraction packet with the key on moodle.
The next scribe is..
Suvd D
Since the notes do not include the ionization of energy and electronegativity, look to the textbook pages 155-156 to help
ReplyDelete