Scribe Post: Juliette Ovadia
Agenda
First, Mrs. Friedmann checked in our homework. The homework was two worksheets, the Intermolecular Forces table and the eight questions on the Intermolecular forces worksheet. Next, we had a discussion about Solar Roadways which was brought up yesterday and other such innovations. We then went over the homework and Mrs. Friedmann answered our questions about the worksheets, web assigns, and review packet. We were supposed to also complete a lab, the Physical Properties of Liquids Lab, but we did not have time to complete it.
Class Discussion
More information can be found on this website: http://www.solarroadways.com/intro.shtml, and the video above is a short documentary about the innovation.
We also discussed the idea that it is the small solutions that make the greatest impact. For instance, in third-world and developing countries especially in Africa, people have to trek for miles carrying heavy tanks to get water, and usually those making that trek are women. The Hippo Water Roller was invented as an easier, less taxing way of carrying water. The video above details the innovation.
 We also discussed solar power, and how we can innovate so that we are not so reliant on fossil fuels. Illinois uses a lot of nuclear energy, yet even nuclear energy comes with its own problems. There is a very interesting growing field called biophotovoltaics, which is working on artificial photosynthesis and how we can harness the power of the sun and plants for solar power. Specifically, a recent paper by Andreas Mershin and some researchers at MIT details how Photosystem I can be aggregated from discarded crops or timber, stabilized by some chemicals, and spread out on a "nanoforest" of semi-conducting electrodes of ZnO nanowires and TiO2 nanostructure that can produce an electric current when exposed to light. This could mean that in a few years people in third-world and developing countries could use discarded plants, a bag of chemicals and some written instructions and paint this/install this on their roof and have solar power. Right now the efficiency is only at 0.1-0.8% but the field is clearly advancing and such an innovation would be extremely useful and life-changing in developing countries. Click here for the link to the paper on this biophotovoltaics innovation
We also discussed solar power, and how we can innovate so that we are not so reliant on fossil fuels. Illinois uses a lot of nuclear energy, yet even nuclear energy comes with its own problems. There is a very interesting growing field called biophotovoltaics, which is working on artificial photosynthesis and how we can harness the power of the sun and plants for solar power. Specifically, a recent paper by Andreas Mershin and some researchers at MIT details how Photosystem I can be aggregated from discarded crops or timber, stabilized by some chemicals, and spread out on a "nanoforest" of semi-conducting electrodes of ZnO nanowires and TiO2 nanostructure that can produce an electric current when exposed to light. This could mean that in a few years people in third-world and developing countries could use discarded plants, a bag of chemicals and some written instructions and paint this/install this on their roof and have solar power. Right now the efficiency is only at 0.1-0.8% but the field is clearly advancing and such an innovation would be extremely useful and life-changing in developing countries. Click here for the link to the paper on this biophotovoltaics innovation
Homework
We went over both of the worksheets in class today. Important things to remember about intermolecular forces:
- Nonpolar--> main IMF London Dispersion Forces even though LDF is at work in every molecule (symmetrical)
- Polar --> main IMF Dipole-Dipole (assymetrical)
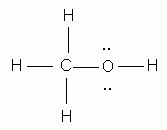
- Usually, if polar w/out a hydrogen attracted to O, N, F (FON) --> dipole/dipole
- If Nonpolar --> London Dispersion Forces
- Polar w/ H attracted to F, O, or N--> Hydrogen Bond
- In addition, these intermolecular forces are on a continuum. We learn the extremes, and the muddy area is for experimentation. H Bond (strongest) <----------> London Dispersion Forces (weakest)

On the second worksheet with the table, the answer to the motor oil question is as follows on the key:
Since motor oil is needed to quickly coat all surfaces of an engine to keep them from getting too hot, a low viscosity material is better than a high viscosity material.
*Viscosity= measure of how well substances flow
*contains H-bond which means high velocity, motor oil has LDF only so it has low viscosity
We also went over sigma and pi bonds, that a pi bond is the last pair of electrons for example on the S in SO2 which spreads out and balances the structure. A pi bond is ultimately an area of space electrons can occupy, orbitals available. The key is that electrons don't stay stuck in between 2 atoms in a pi bond, if there is an empty pi bond they will smear onto it and occupy the entire space, and we show resonance to demonstrate this.
Had we done the lab, we would have worked with:
1) Hexane --> no polarity so main IMF is LDF and would evaporate quickly and have a low boiling point
 |
| Hexane |
2) Vegetable Oil --> LDF and Dipole-dipole and polarity, but the predominant intermolecular force is LDF
3) Water --> Hydrogen bonding
Homework
A good way to study would be to work on the review packet. The key is posted on moodle.
The next scribe post author is: Valerie Korol
No comments:
Post a Comment